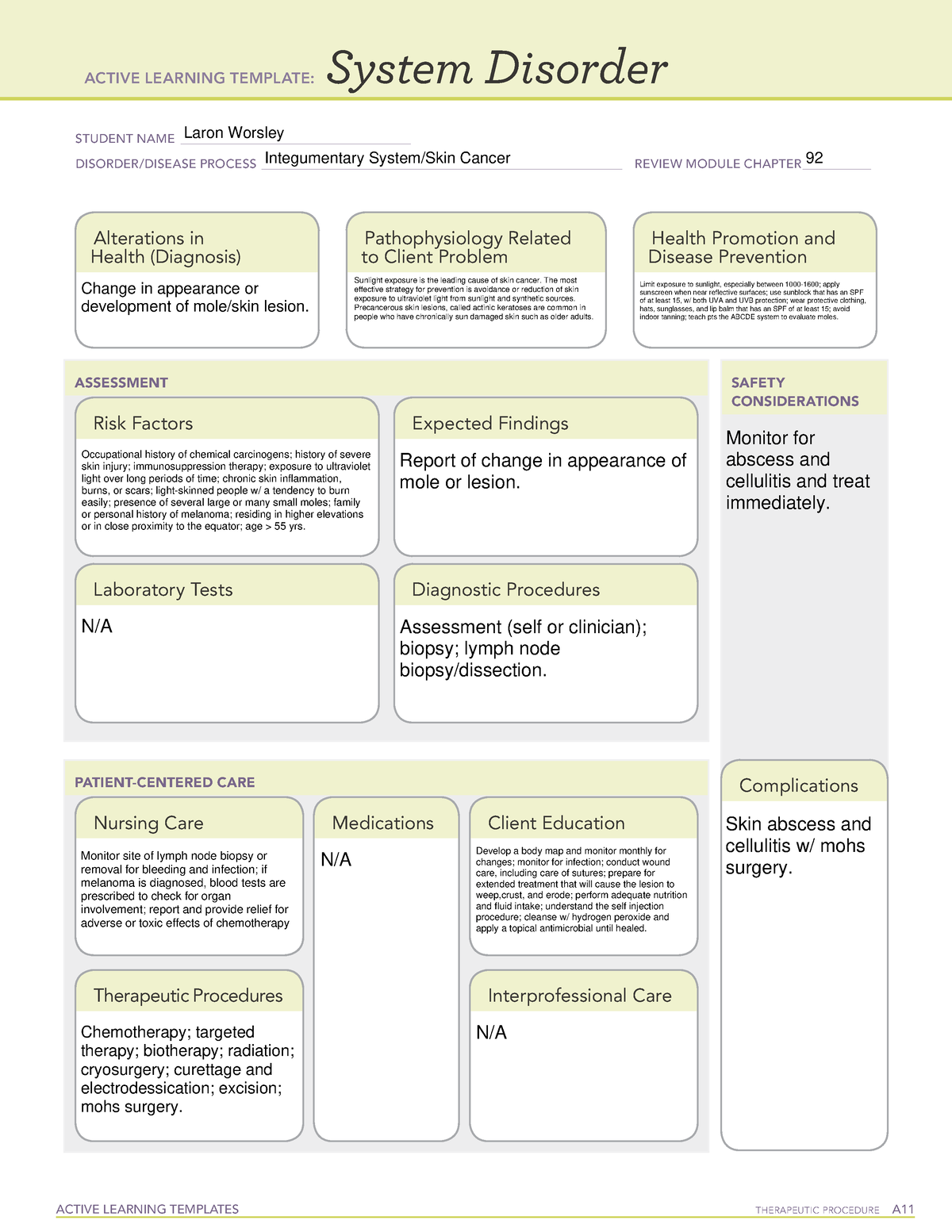
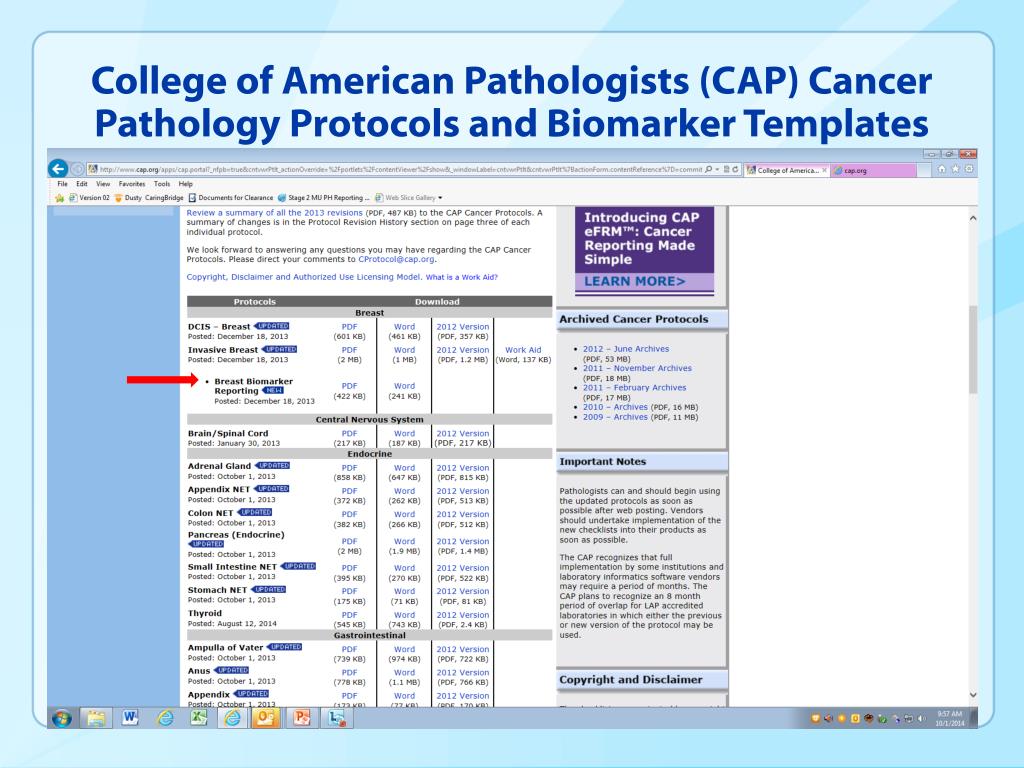
Cap Tumor Template
Cap Tumor Template - The electronic versions of over 100 cancer protocols and cancer biomarker templates help pathologists create high quality cancer reports within their current system workflows. Findings revealed that most of the approximately 160 respondents (87 percent) use the cap cancer protocols, four percent use the international collaboration on cancer reporting (iccr). The list of mandated cap cancer checklists and effective dates can be found in appendix 4.1. To date, cco pathology laboratory medicine program mandated use of 6 cap biomarker. For reporting molecular testing and immunohistochemistry for mismatch repair proteins, and for other cancer biomarker testing results, the cap colorectal biomarker template should be. Completion of the template is the responsibility of the. For reporting cancer biomarker testing results, the cap lung biomarker template may be used. For accreditation purposes, only the definitive primary cancer resection specimen is required to have the core and conditional data elements reported in a synoptic format. Cap cancer protocol templates provide guidelines for collecting the essential data elements for complete reporting of malignant tumors and optimal patient… For accreditation purposes, only the definitive primary cancer resection specimen is required to have the core and conditional data elements reported in a synoptic format. From the college of american pathologists, american society for clinical pathology, and american society of clinical oncology. For reporting cancer biomarker testing results, the cap lung biomarker template may be used. Pending biomarker studies should be listed in the comments section of this report. Surgery, radiation, and chemotherapy are standard therapeutic options for brain tumors. The html form and cascade style sheet were created on pform , with references to javascripts and. View current protocols and download templates. The list of mandated cap cancer checklists and effective dates can be found in appendix 4.1. For accreditation purposes, only the definitive primary cancer resection specimen is required to have the core and conditional data elements reported in a synoptic format. For accreditation purposes, only the definitive primary cancer resection specimen is required to have the core and conditional data elements reported in a synoptic format. Choose from digital or print/pdf versions to help your laboratory achieve better patient outcomes. Cancer protocols and biomarker reporting templates; For accreditation purposes, only the definitive primary cancer resection specimen is required to have the core and conditional data elements reported in a synoptic format. This allows anatomical pathologists to report on a far greater number of cases while. For reporting molecular testing and immunohistochemistry for mismatch repair proteins, and for other cancer biomarker. Pending biomarker studies should be listed in the comments section of this report. Surgery, radiation, and chemotherapy are standard therapeutic options for brain tumors. To promote understanding of the college of american pathologists (cap) cancer protocols and their critical use in cancer patient care, and advance collaboration and. View current protocols and download templates. Completion of the template is the. The electronic versions of over 100 cancer protocols and cancer biomarker templates help pathologists create high quality cancer reports within their current system workflows. To date, cco pathology laboratory medicine program mandated use of 6 cap biomarker. For accreditation purposes, only the definitive primary cancer resection specimen is required to have the core and conditional data elements reported in a. Surgery, radiation, and chemotherapy are standard therapeutic options for brain tumors. Completion of the template is the responsibility of the. Cap cancer protocol templates provide guidelines for collecting the essential data elements for complete reporting of malignant tumors and optimal patient… For accreditation purposes, only the definitive primary cancer resection specimen is required to have the core and conditional data. Access the new autopsy protocol; To date, cco pathology laboratory medicine program mandated use of 6 cap biomarker. This allows anatomical pathologists to report on a far greater number of cases while. From the college of american pathologists, american society for clinical pathology, and american society of clinical oncology. Surgery, radiation, and chemotherapy are standard therapeutic options for brain tumors. For accreditation purposes, only the definitive primary cancer resection specimen is required to have the core and conditional data elements reported in a synoptic format. For accreditation purposes, only the definitive primary cancer resection specimen is required to have the core and conditional data elements reported in a synoptic format. These updated ecc's include new fields, terms, options, titles, and. Choose from digital or print/pdf versions to help your laboratory achieve better patient outcomes. Cap cancer protocol templates provide guidelines for collecting the essential data elements for complete reporting of malignant tumors and optimal patient… For reporting cancer biomarker testing results, the cap lung biomarker template may be used. These updated ecc's include new fields, terms, options, titles, and even. View current protocols and download templates. Completion of the template is the responsibility of the. For reporting cancer biomarker testing results, the cap lung biomarker template may be used. Findings revealed that most of the approximately 160 respondents (87 percent) use the cap cancer protocols, four percent use the international collaboration on cancer reporting (iccr). Cancer protocols and biomarker reporting. Choose from digital or print/pdf versions to help your laboratory achieve better patient outcomes. Cancer protocols and biomarker reporting templates; Cancer biomarker testing results, the cap colorectal biomarker template should be used. For accreditation purposes, only the definitive primary cancer resection specimen is required to have the core and conditional data elements reported in a synoptic format. From the college. These updated ecc's include new fields, terms, options, titles, and even new templates to be used. The html form and cascade style sheet were created on pform , with references to javascripts and. To date, cco pathology laboratory medicine program mandated use of 6 cap biomarker. Cap cancer protocol templates provide guidelines for collecting the essential data elements for complete. For accreditation purposes, only the definitive primary cancer resection specimen is required to have the core and conditional data elements reported in a synoptic format. For reporting cancer biomarker testing results, the cap lung biomarker template may be used. Choose from digital or print/pdf versions to help your laboratory achieve better patient outcomes. For accreditation purposes, only the definitive primary cancer resection specimen is required to have the core and conditional data elements reported in a synoptic format. The html form and cascade style sheet were created on pform , with references to javascripts and. For reporting molecular testing and immunohistochemistry for mismatch repair proteins, and for other cancer biomarker testing results, the cap colorectal biomarker template should be. The electronic versions of over 100 cancer protocols and cancer biomarker templates help pathologists create high quality cancer reports within their current system workflows. View current protocols and download templates. To promote understanding of the college of american pathologists (cap) cancer protocols and their critical use in cancer patient care, and advance collaboration and. Cap cancer protocol templates provide guidelines for collecting the essential data elements for complete reporting of malignant tumors and optimal patient… This allows anatomical pathologists to report on a far greater number of cases while. Access the new autopsy protocol; To date, cco pathology laboratory medicine program mandated use of 6 cap biomarker. For accreditation purposes, only the definitive primary cancer resection specimen is required to have the core and conditional data elements reported in a synoptic format. Cancer biomarker testing results, the cap colorectal biomarker template should be used. Surgery, radiation, and chemotherapy are standard therapeutic options for brain tumors.Cap Cancer Template prntbl.concejomunicipaldechinu.gov.co
Cap Protocol Templates
CAP Cancer Protocol Esophagus Doc Template pdfFiller
Cap Cancer Templates
Cap Cancer Template
Cap Cancer Templates
Cap Cancer Templates
CAP Cancer Protocol Intrahepatic Bile Ducts Doc Template pdfFiller
Cap Cancer Template
PPT Cancer Pathology and Biomarker Reporting PowerPoint Presentation
Completion Of The Template Is The Responsibility Of The.
These Updated Ecc's Include New Fields, Terms, Options, Titles, And Even New Templates To Be Used.
From The College Of American Pathologists, American Society For Clinical Pathology, And American Society Of Clinical Oncology.
Findings Revealed That Most Of The Approximately 160 Respondents (87 Percent) Use The Cap Cancer Protocols, Four Percent Use The International Collaboration On Cancer Reporting (Iccr).
Related Post: