Iq Oq Pq Template
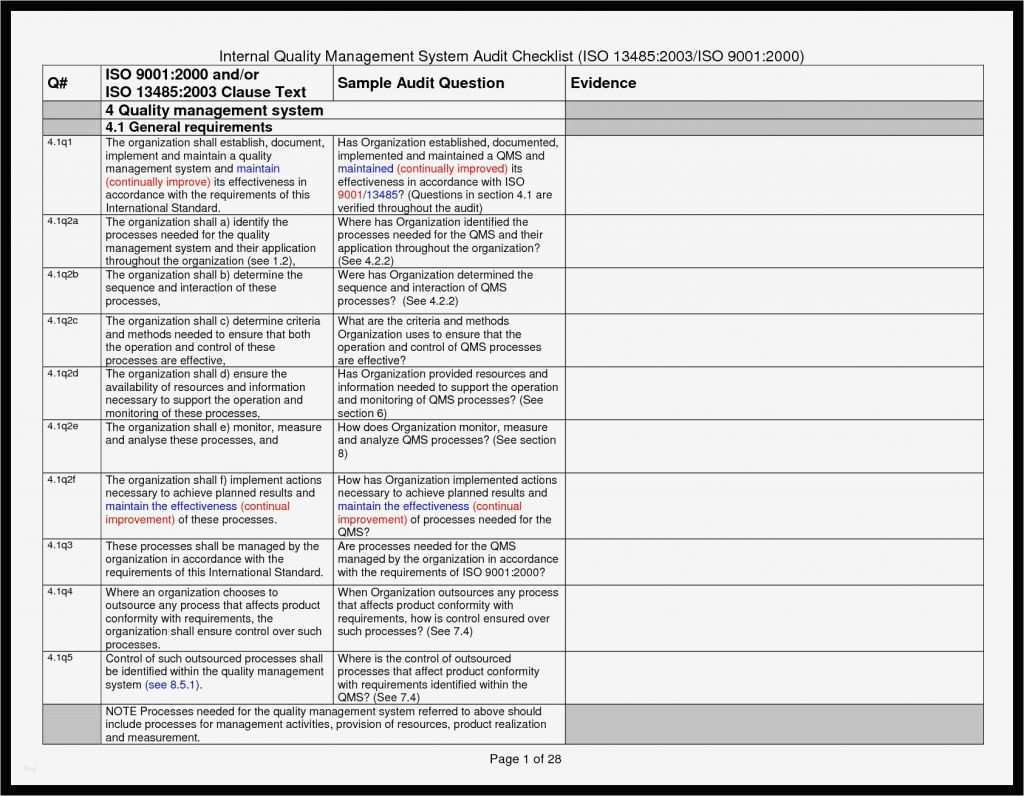
Iq Oq Pq Template - Performance qualification (pq) demonstrate the process will consistently produce acceptable product under normal operating conditions. Things to consider… • approved procedures and. The objective of this protocol is to define the installation qualification (iq) and operational qualification (oq) requirements and acceptance criteria for the [insert system name and plant. This template is suitable for authoring the tests of either user requirements (pq) or functional requirements (oq) template sections include: It also produces the thorough audit trail needed to meet all. You can use this for a full qualification, add or remove any sections as you require. Does on average, reduce protocol authoring, and execution approval times by 40%. The combined qualification has been carefully designed. It covers the documentation of iq/oq/pq. Find out the best practices, challenges, and tips for. Iq oq pq medical devices process validation is conducted to ensure consistent delivery of quality products meeting its predetermined specifications and quality characteristics. The combined qualification has been carefully designed. Find out the best practices, challenges, and tips for. This is a combination of the iq, oq, and pq. It covers the documentation of iq/oq/pq. Does on average, reduce protocol authoring, and execution approval times by 40%. The intent of this dq/iq/oq/pq protocol is to define and assure the implementation of the organizational practices, standards, methods, and documentation conventions to be used for. Learn what iq, oq, pq are and how to perform them in pharmaceutical, medical devices, and clinical industries. You can use this for a full qualification, add or remove any sections as you require. Learn about iq oq pq validation processes, iq oq pq examples, and essential iq oq templates to ensure quality in your manufacturing operations. This sop applies to all iq, oq, and pq activities performed on pharmaceutical manufacturing equipment, systems, and processes. It covers the documentation of iq/oq/pq. Does on average, reduce protocol authoring, and execution approval times by 40%. Learn what iq, oq, pq are and how to perform them in pharmaceutical, medical devices, and clinical industries. This template is suitable for authoring. The intent of this dq/iq/oq/pq protocol is to define and assure the implementation of the organizational practices, standards, methods, and documentation conventions to be used for. The objective of this protocol is to define the installation qualification (iq) and operational qualification (oq) requirements and acceptance criteria for the [insert system name and plant. Performance qualification (pq) demonstrate the process will. This sop applies to all iq, oq, and pq activities performed on pharmaceutical manufacturing equipment, systems, and processes. You can use this for a full qualification, add or remove any sections as you require. The combined qualification has been carefully designed. It also produces the thorough audit trail needed to meet all. Iq oq pq medical devices process validation is. This template is suitable for authoring the tests of either user requirements (pq) or functional requirements (oq) template sections include: It covers the documentation of iq/oq/pq. Find out the best practices, challenges, and tips for. Does on average, reduce protocol authoring, and execution approval times by 40%. Learn about iq oq pq validation processes, iq oq pq examples, and essential. Performance qualification (pq) demonstrate the process will consistently produce acceptable product under normal operating conditions. You can use this for a full qualification, add or remove any sections as you require. Iq oq pq medical devices process validation is conducted to ensure consistent delivery of quality products meeting its predetermined specifications and quality characteristics. Does on average, reduce protocol authoring,. Find out the best practices, challenges, and tips for. It also produces the thorough audit trail needed to meet all. Iq oq pq medical devices process validation is conducted to ensure consistent delivery of quality products meeting its predetermined specifications and quality characteristics. Performance qualification (pq) demonstrate the process will consistently produce acceptable product under normal operating conditions. The combined. This template is suitable for authoring the tests of either user requirements (pq) or functional requirements (oq) template sections include: Learn about iq oq pq validation processes, iq oq pq examples, and essential iq oq templates to ensure quality in your manufacturing operations. The objective of this protocol is to define the installation qualification (iq) and operational qualification (oq) requirements. This template is suitable for authoring the tests of either user requirements (pq) or functional requirements (oq) template sections include: You can use this for a full qualification, add or remove any sections as you require. It also produces the thorough audit trail needed to meet all. It covers the documentation of iq/oq/pq. Find out the best practices, challenges, and. You can use this for a full qualification, add or remove any sections as you require. This is a combination of the iq, oq, and pq. Things to consider… • approved procedures and. It also produces the thorough audit trail needed to meet all. This template is suitable for authoring the tests of either user requirements (pq) or functional requirements. The combined qualification has been carefully designed. Things to consider… • approved procedures and. Find out the best practices, challenges, and tips for. Learn what iq, oq, pq are and how to perform them in pharmaceutical, medical devices, and clinical industries. This template is suitable for authoring the tests of either user requirements (pq) or functional requirements (oq) template sections. The combined qualification has been carefully designed. Things to consider… • approved procedures and. It covers the documentation of iq/oq/pq. Iq oq pq medical devices process validation is conducted to ensure consistent delivery of quality products meeting its predetermined specifications and quality characteristics. The objective of this protocol is to define the installation qualification (iq) and operational qualification (oq) requirements and acceptance criteria for the [insert system name and plant. Learn what iq, oq, pq are and how to perform them in pharmaceutical, medical devices, and clinical industries. The intent of this dq/iq/oq/pq protocol is to define and assure the implementation of the organizational practices, standards, methods, and documentation conventions to be used for. Does on average, reduce protocol authoring, and execution approval times by 40%. Find out the best practices, challenges, and tips for. This sop applies to all iq, oq, and pq activities performed on pharmaceutical manufacturing equipment, systems, and processes. Performance qualification (pq) demonstrate the process will consistently produce acceptable product under normal operating conditions. Learn about iq oq pq validation processes, iq oq pq examples, and essential iq oq templates to ensure quality in your manufacturing operations.Iq Oq Pq Templates
Iq Oq Pq Templates Download 4 Free Professional Templates Images
What is IQ, OQ, PQ? [Quick Guide to Process Validation]
Iq Oq Pq Vorlage Schön Iopq Freezer Validation Template Sample by
Six Sigma Validation Process IQ Installation Qualification OQ
Iq Oq Pq Vorlage Schön 10 New Iq Oq Pq Validation Templates
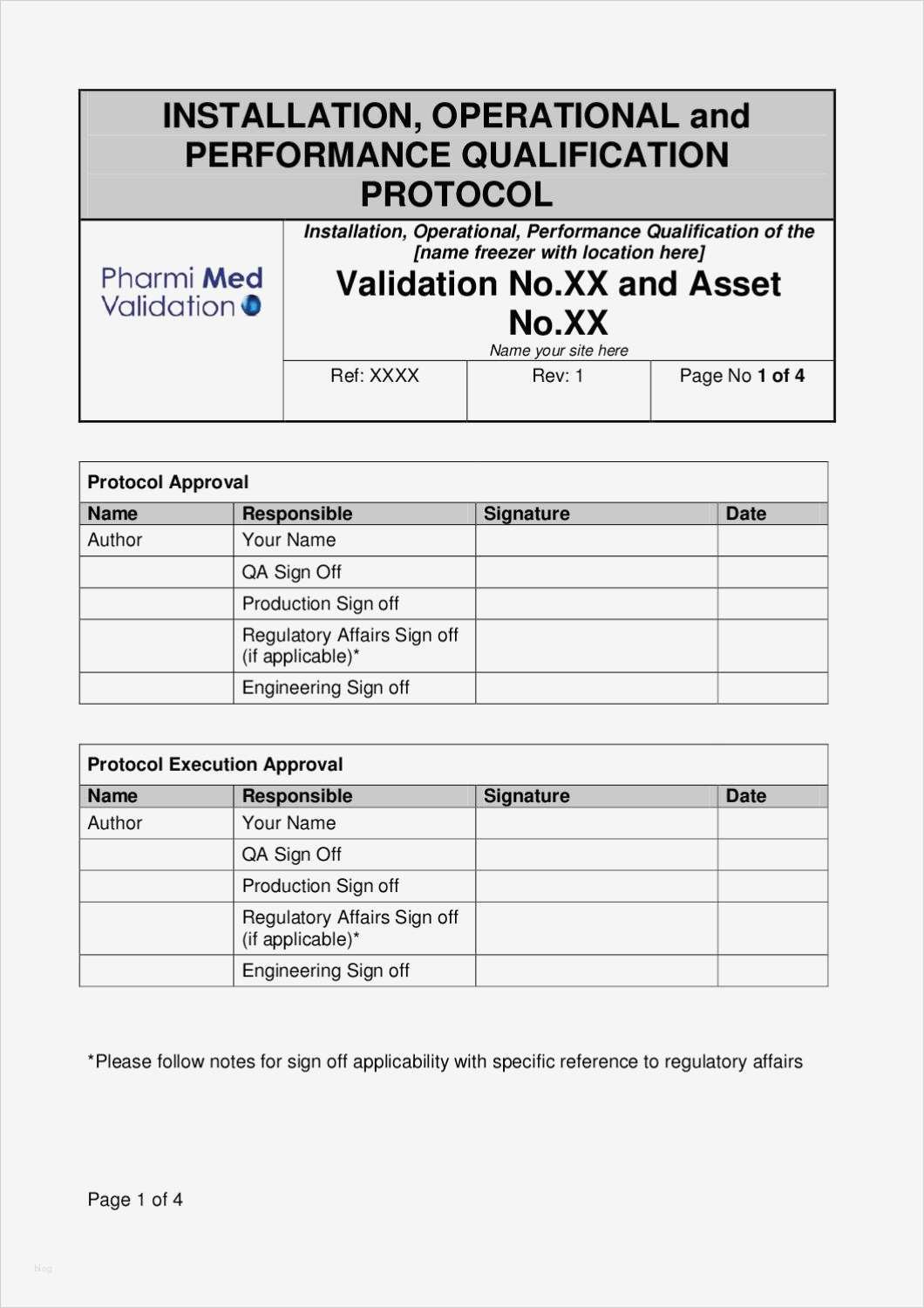
Iq Oq Pq Template
Iq Oq Pq Template
Iq Oq Pq Templates
Iq Oq Pq Templates
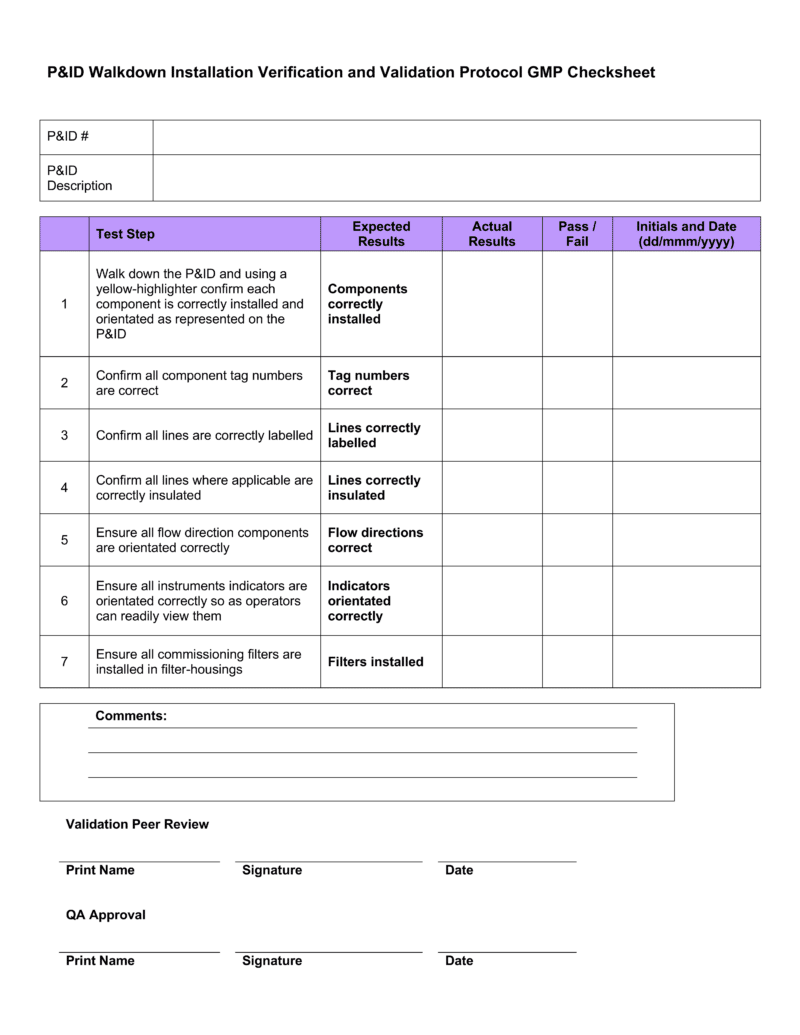
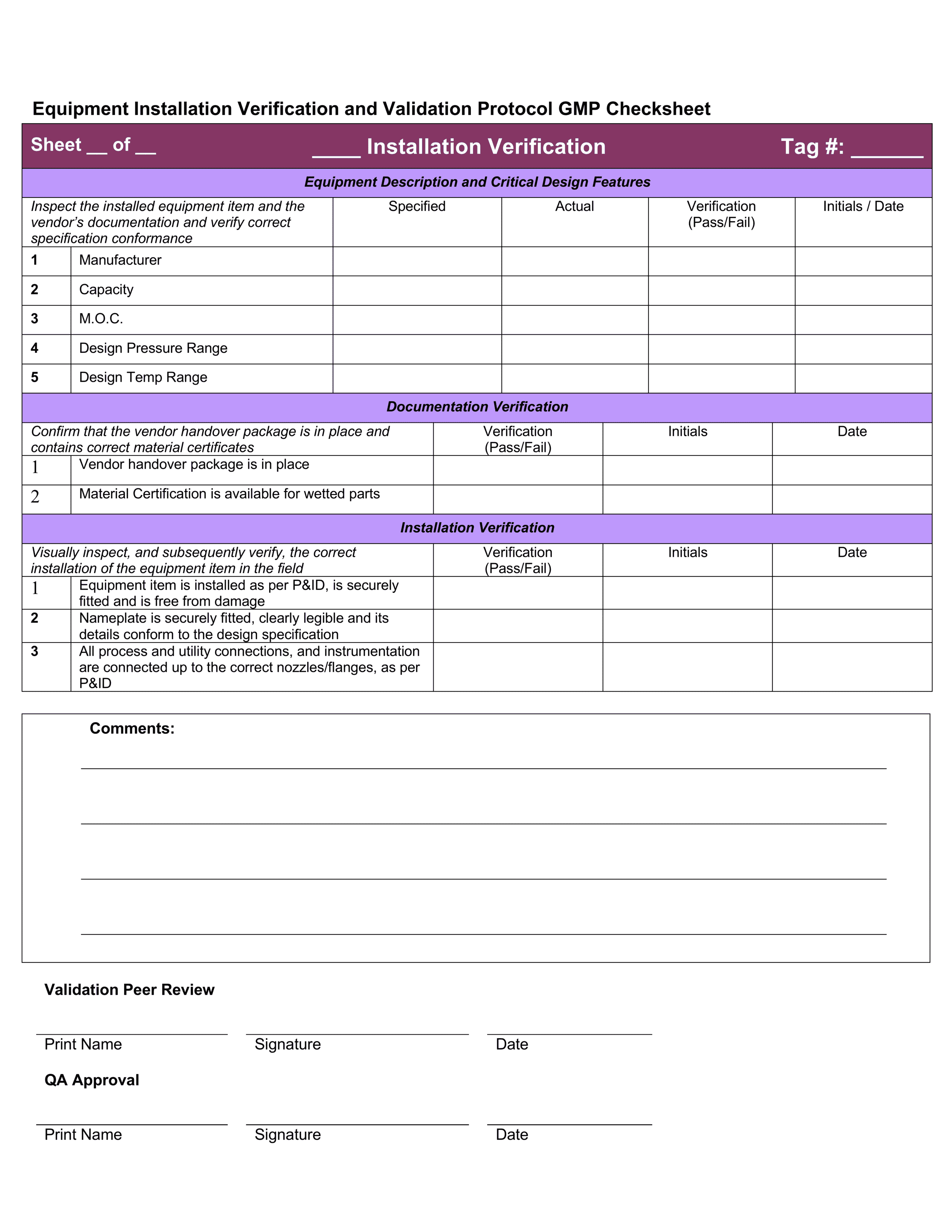
This Is A Combination Of The Iq, Oq, And Pq.
This Template Is Suitable For Authoring The Tests Of Either User Requirements (Pq) Or Functional Requirements (Oq) Template Sections Include:
You Can Use This For A Full Qualification, Add Or Remove Any Sections As You Require.
It Also Produces The Thorough Audit Trail Needed To Meet All.
Related Post:
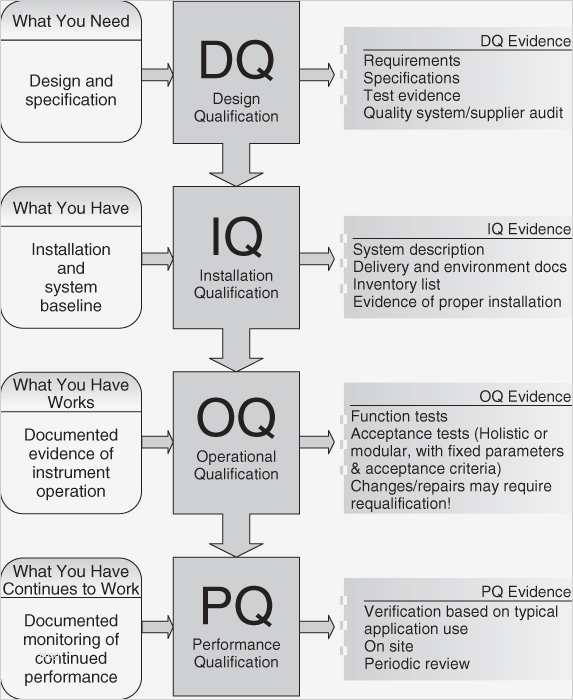

![What is IQ, OQ, PQ? [Quick Guide to Process Validation]](https://blog.greenlight.guru/hubfs/IQ OQ PQ.png)